Hello I'm Vincent the Science Rep and I just very bo liao so let's revise CHEMISTRY=D
First is the element. An element is a pure substance that cannot be broken down into any simpler substance. There are 109 elements in total . Anyway they are all arranged in the periodic table.
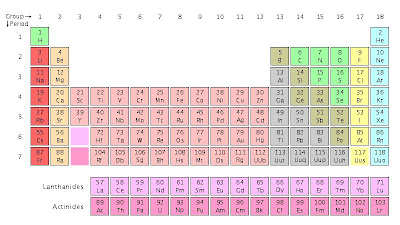 (Click for larger image. Taken from Wikipedia www.wikipedia.org)
(Click for larger image. Taken from Wikipedia www.wikipedia.org)Group=A column(vertical) in the periodic table. For example,beryllium, magnesium, calcium are all in the same group. Elements in the same group have similar properties
Period=A row(horizontal) in the periodic table. Potassium, calcium, scandium and titanium are in the same period. A period shows a gradual change from metallic properties(left) to non-metallic properties(right).
Properties of metallic elements:Good conductor of heat & electricity, ductile(can be pulled into wires), malleable(can be beaten into any shape), shiny, have high densities...
Properties of non-metallic elements: Poor conductor of heat & electricity, dull, have low densities, brittle(solids)
IMPORTANTZ: I cannot gurantee that all the metallic and non-metallic elements have all these properties.
Compounds and MixturesA compound is a substance made up of two or more elements chemically combined together. Here are some common compounds found in our everyday life: Water=hydrogen and oxygen, salt=sodium and chloride, sugar=carbon,hydrogen and oxygen.
A mixture is a substance made up of two or more substances chemically combined together. Got lots of mixtures which will take up a lot of time so go read your textbooks and worksheets.
Properties of Compounds and MixturesWell I have made a 'picture' showing all the properties of compounds and mixtures. Click on it to enlarge.
 Seperation of Mixtures
Seperation of MixturesYes'd finally we are reaching the final topic. There are 5 ways to separate mixtures. They are magnetic attraction, filtration, evaporation, distillation and chromatography.
1)Magnetic Attraction
It is used to seperate magnetic substances from non-magnetic substances
2)Filtration
Filtration is used to seperate an insoluble solid from a liquid. For example, sand from water.
Diagram:
 (Click to enlarge)
(Click to enlarge)3)Evaporation
This is used to seperate a soluble solid from a liquid. Example=Salt solution
4)Distillation
This is used to seperate liquid from a solution. Example=Seawater
 (Click to enlarge. Taken from Wikipedia www.wikipedia.org)
(Click to enlarge. Taken from Wikipedia www.wikipedia.org)5)Chromatography
This is used to seperate dyes from inks/colours
If you spot a mistake, please tell me=D=D=D





